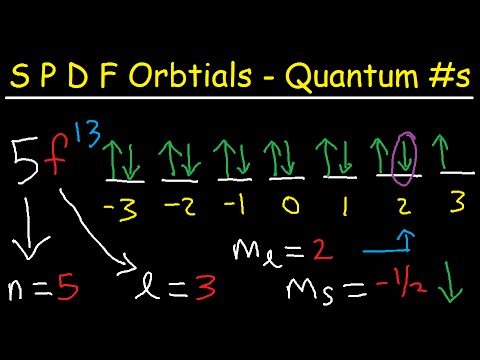
In this video, we're going to talk about the SPD F sublevels. One thing we need to know is that S has a spherical shape, just like a sphere. P has a dumbbell shape, which can be drawn in two ways. D is like a cloverleaf, and F has some unusual shape that varies. Some things you need to know is that the number of energy levels is equal to the number of sublevels. So, when n is 1, you only have 1 sublevel, which is S. When n is 2, you have two sublevels, S and P. When n is 3, you have 3 sublevels, 3s, 3p, and 3d. When n is 4, there are 4 sublevels, 4s, 4p, 4d, and 4f. The S sublevel can hold up to two electrons, and you need to know that every orbital can hold up to two electrons. So S has one orbital. Now, in a periodic table, the S block is the first two columns, Group one and Group two. P can hold up to 6 electrons, and if you notice the P block in the periodic table, it's from Group 13 to Group 18. So, it can hold up to 6 electrons and has three orbitals. D can hold up to 10 electrons, and the elements in the D block, like zinc, copper, and nickel, are in the 3d sublevel. There are 10 elements in the D block, and it has 5 orbitals. F can hold up to 14 electrons and has 7 orbitals. So, those are some things you want to keep in mind. By the way, whenever you have the S sublevel, L is equal to 0. For the P sublevel, L is equal to 1. For D, L is equal to 2. And for F, L is...
Award-winning PDF software





Video instructions and help with filling out and completing Fill Form 8815 Amounts