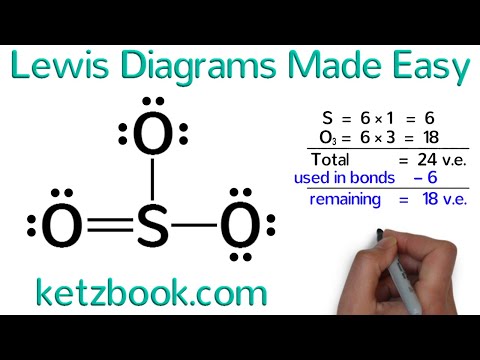
Welcome to Lewis diagrams made easy with Ketz book. Today, we're going to learn how to draw Lewis diagrams for atoms and simple molecules. But before we begin, let's start with the question: how many valence electrons does chlorine have? In order to answer that question, we need to look at the periodic table. Remember that within any given column, all the elements have the same number of valence electrons. To get that number, all we need to do is count the columns starting from the left, skip the transition metals, and remember that the only exception to this is helium, which has only two valence electrons, not eight. Now, find chlorine in the periodic table. Remember that its symbol is Cl and it is in the seventh column. That tells us that it has seven valence electrons. Knowing the number of valence electrons an element has is critical, and in Lewis diagrams, we use dots to represent valence electrons. So, the Lewis diagram of chlorine is the symbol Cl with seven dots around it. When you draw the dots, don't just put them anywhere. Instead, imagine a square around the element symbol. The dots should be neatly drawn on the four sides of the square, with no more than two dots on any side. Practice drawing Lewis diagrams with a few elements just to make sure you've got it. This is the Lewis diagram of hydrogen, which has only one valence electron. This is carbon, which has four valence electrons. And this is oxygen, which has six valence electrons. Where you put the dots doesn't really matter, as long as you neatly draw them along the sides of an imaginary square and never put more than two dots on one side. Lewis diagrams are often used to represent covalent bonding in molecules and ions....
Award-winning PDF software





Video instructions and help with filling out and completing How Form 8815 Representation